BEGINNING AT BIRTH, THE crystalline lens undergoes progressive changes in which the lens gradually becomes thicker, less flexible, and opaque. These changes result in losses in lens accommodation and optical quality that first manifest clinically as presbyopia in a person’s 40s, and as visually significant cataracts in later life.
The global impact of cataracts on quality of life is massive. Patients with visually significant cataracts have an increased risk of falls and accidents, and vision impairment is associated with depression, cognitive difficulties, social isolation, and higher mortality rates. While blindness due to cataracts is rare in the United States, 15.2 million people worldwide are blind from cataracts, and another 78.8 million have moderate to severe vision impairment from cataracts.1 In low- and middle-income countries, cataracts are the leading cause of blindness, and access to cataract surgery is severely limited.2 Even in the United States, as the number of patients with cataract grows from the current 24.4 million to 45.6 million by 2050,3 it will be challenging to fully meet the need with the current supply of ophthalmologists.
In the United States, cataract extraction with intraocular lens (IOL) implantation is the most common surgical procedure performed and among the most successful. Cataract surgery significantly improves visual function and quality of life, especially when both eyes have been operated on,4 but it does not restore the performance of a youthful lens, nor is it risk-free. A very few patients may experience retinal complications, such as macular edema, retinal detachment, or rarely postoperative endophthalmitis. More commonly, posterior capsular opacification can occur, requiring laser capsulotomy. Further, some patients experience visual complaints after surgery, ranging from reduced depth of focus to various forms of dysphotopsias. Despite all our technological advancements with premium IOLs, we still cannot match the performance of a young, crystalline lens.
What if we could delay or prevent the loss in vision associated with the aging lens from occurring in the first place?
Novartis attempted to preserve youthful lens elasticity with a drug called UNR844, a lipoic acid choline ester delivered via a topical eye drop. The proposed mechanism of action was the reversal of disulfide bonds in the lens, restoring elasticity to treat presbyopia and potentially cataract itself.5 Although early phase 1/2 studies of UNR844 were promising, the drug failed to meet its primary endpoint in a recent phase 2b study, leading Novartis to announce that it was abandoning development of the compound. It is not clear whether topical administration was able to achieve therapeutic drug concentrations in the human lens to achieve the intended effect.
Despite these disappointing results, a nonsurgical treatment that slows or reverses the underlying processes of lens aging, even if intraocular injection is required to achieve satisfactory results, could be game changing.
The latest hope to modulate lens aging processes and address cataract via nonsurgical means comes from a library of novel compounds recently acquired by Visus Therapeutics from ViewPoint Therapeutics. Visus already has a pupil-modulating therapy, Brimochol PF, in late-stage development for presbyopia. A lens-modulating therapy would be complementary to the company’s pipeline and of great interest to cataract and refractive surgeons.
Mechanism of Action
The clear crystalline lens is made up of elongated fiber cells containing high concentrations of crystallin proteins (Figure 1). There are 3 major types of crystallin present in the lens: α-, β- and γ-crystallins. These proteins must last for our entire lifetime since they cannot regenerate. The most common is α-crystallin, which acts as a chaperone for the other crystallins, binding to unfolded and damaged proteins before they can aggregate into complexes that cloud the lens. These chaperone crystallins do much of the work to keep the lens fibers organized, flexible, and clear. As the lens ages, the α-crystallins, which predominate natively in a soluble dimer conformation, break down into monomers and lose their chaperone activity, leading to aggregation of the surrounding proteins.
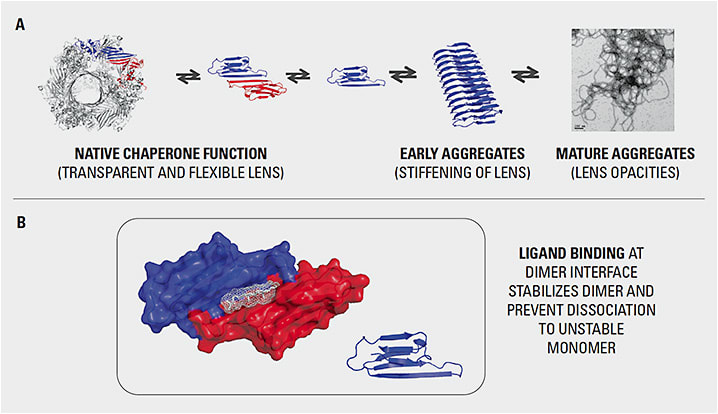
Early research in mice demonstrated that a small-molecule compound could restore the dimer conformation of the α-crystallin, which could once again solubilize the β- and γ-crystallins. This molecule had the effect of partially restoring transparency in the mouse model.6 Exogenous chaperones have also been shown to reverse aggregation in aged human lens homogenates.7
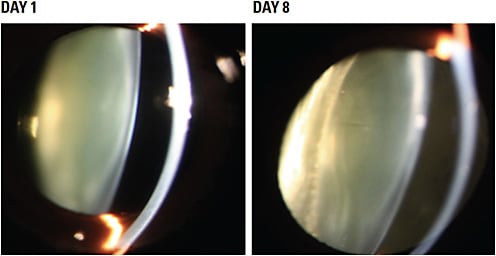
Visus recently reported proof of concept in restoring lens clarity using an α-crystallin chaperone delivered by intravitreal injection in nonhuman primates with spontaneous, age-related nuclear cataracts, which are similar to human cataracts. In that unpublished study, senior cynomolgus monkeys were screened and graded for nuclear cataract at the slit lamp using the Lens Opacities Classification System8 (LOCS III) transparency with standardized lighting. Eight monkeys aged 15 to 22 years old, with LOCS III scores ≥2.5, were selected. The treated eye was dosed with an intraocular suspension of 25-hydroxy cholesterol (25-HC), a known α-crystalline chaperone, while the untreated, fellow eye was not dosed. A clinically meaningful reduction in lens opacity was seen as early as 8 days after injection (Figure 2), with the effects of a single dose lasting for 57 days (Figure 3).
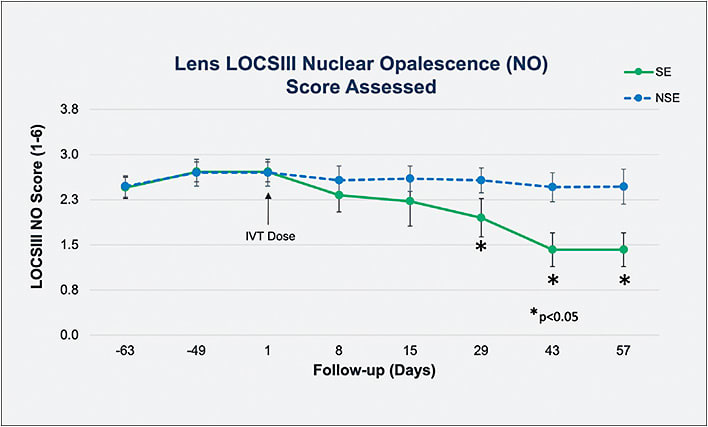
Despite its good activity, 25-HC is not a viable drug development candidate. As part of its VT-1061 lens modulation program, Visus is developing a second generation α-crystallin with excellent pharmaceutical properties that stabilizes native α-crystallin and restores its natural chaperone activity, with the goal of preventing and reversing the protein aggregation that causes the loss in vision.
There is, of course, much work to be done. Pre-clinical work is ongoing, with investigational new drug (IND) filing targeted for Q4 2024.
Future Impact
A nonsurgical treatment for cataract could be transformational for eye health worldwide. We are still years away from understanding how lens-modulating drugs could work in the clinical setting and who would be the ideal target candidates. One might imagine that, in emmetropes, lens modulation might reverse the visual disturbance caused by an early cataract, restore some accommodation, and postpone the need for cataract surgery, possibly indefinitely. For myopes with early cataract, lens modulation that clears the lens and restores some accommodation could make these patients refractive surgery candidates to address their refractive error. For postrefractive patients, lens modulation could restore useful accommodation when they present in their 40s with the complaint that their LASIK “wore off.”
There are also many barriers to successfully bringing to market a lens-modulating therapy. One obvious question comes to mind: if effective treatment of dysfunctional lens syndrome does require intraocular delivery, would anterior segment surgeons create injection clinics to manage these cases, just as our retina colleagues successfully did? Would patients participate?
Although there is still much to learn, this is a promising technology, and I encourage my colleagues to keep an open mind as we follow its development and contemplate how a nonsurgical therapy could fit in with modern cataract surgery. We should approach the science critically but unselfishly, and welcome innovation with the potential to improve patients’ lives and reduce cataract blindness around the world. ■
References
- Pesudovs K, Lansignh VC, Kempen JH, et al. Cataract-related blindness and vision impairment in 2020 and trends over time in relation to VISION 2020: The Right to Sight: An analysis for the Global burden of Disease Study. Invest Ophthalmol Vis Sci. 2021;62:3523.
- Liu YC, Wilkins M, Kim T, et al. Cataracts. Lancet. 2017;390(10094):600-612.
- The burden of vision loss. Vision Health Initiative website, Centers for Disease Control and Prevention. Accessed November 2, 2022. https://www.cdc.gov/visionhealth/risk/burden.htm
- Shekhawat NS, Stock MV, Baze EF, et al. Impact of first eye versus second eye cataract surgery on visual function and quality of life. Ophthalmology. 2017;124(10):1496-1503.
- Korenfeld MS, Robertson SM, Stein JM, et al. Topical lipoic acid choline ester eye drop for improvement of near visual acuity in subjects with presbyopia: A safety and preliminary efficacy trial. Eye (Lond). 2021;3292-3301.
- Molnar KS, Dunyak BM, Sue B, et al. Mechanism of action of VP1-001 in cryAB(R120G)-associated and age-related cataracts. Invest Ophthalmol Vis Sci. 2019;60(10):3320-3331.
- Makley LN, McMenimen KA, DeVree BT, et al. Pharmacological chaperone for α-crystallin partially restores transparency in cataract models. Science. 2015;350(6261):674-677.
- Chylack LT Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II). Arch Ophthalmol. 1989 Jul;107(7):991-997.









