INTRODUCTION
MIOTICS HAVE BEEN UTILIZED IN EYE CARE FOR MORE THAN 100 years. The most used topical miotic is pilocarpine, which historically served as one of the key intraocular pressure-lowering medications, even for open angle glaucoma, prior to the advent of beta blockers, followed by adrenergic agonists.1 More recently, there has been a renewed interest in miotics as a treatment option for presbyopia, and a variation of 1.25% pilocarpine (Vuity, Allergan/AbbVie) has re-emerged on the market and is commercially available (prescription only) for the treatment of presbyopia.2,3 Other clinical uses of miotics include diagnostic testing of a dilated pupil, reversal of pupillary dilation, decreasing postoperative refractive surgery nighttime glare, and treating angle closure glaucoma.4,5
Understanding the Mechanism Behind Posterior-segment Complications
Pupillary miosis can be achieved by stimulating the iris sphincter muscle or inhibiting the dilator.6 The most effective ocular miotic agents are parasympathomimetic drugs that stimulate the parasympathetic pathway, such as pilocarpine, carbachol, and aceclidine.2,6,7 Agents utilized for their miotic effects that inhibit the dilator and avoid ciliary spasm include phentolamine and brimonidine.2,6,8
Pilocarpine mimics naturally occurring acetylcholine and binds to muscarinic receptors located within the pupillary sphincter and the ciliary body.6 Parasympathomimetic drug-induced smooth muscle contraction of the iris sphincter causes miosis (reduced pupil size), as well as increased depth of focus. Ciliary body contraction results in accommodation, mechanical opening of the trabecular meshwork, and expansion of Schlemm canal.6,9
Posterior-segment complications that occur with miotics are linked to parasympathomimetic medications, and the risk for these complications is directly proportional to the ability of the medication to induce ciliary body spasm.10 There are 2 proposed mechanisms to explain why retinal side effects could occur with parasympathomimetic miotic use (Figure 1).

- Ciliary body contraction causes forward displacement of the posterior lens surface and the vitreous base.4,11-13 These sites are both strong vitreous attachment sites and in turn cause anterior shifting of the vitreous body.4 Anterior displacement of the vitreous, combined with increased axial lengthening, could aggravate pre-existing areas of vitreoretinal traction (vitreoretinal tufts, lattice, stage 1 macular holes, etc.) or create traction at focal regions of firm vitreoretinal attachment.4,13,14
- Contraction of the ciliary body muscle itself, particularly the longitudinal portion, may pull on the peripheral retina, resulting in forward translational movement of the ora serrata.4,13 The longitudinal portion of the ciliary body has a fixed origin at the scleral spur and a potentially mobile insertion within the anterior choroid beneath the ora serrata of the retina.15 When the muscle contracts and shortens, the choroidal insertion moves anteriorly. It is estimated that the ora serrata and underlying choroid move forward approximately 0.05mm for each diopter of accommodation.13 Mechanical pulling in areas of peripheral retinal compromise (thinning or weakness) could lead to the formation of retinal breaks.4
Posterior-segment Complications Reported With Ocular Miotics
The onset of posterior-segment complications from topical miotic use ranges in the literature from 10 hours to 2 months.13,16,17 There have been a few cases of serious complications, such as vitreofoveal traction and retinal detachment, that occurred acutely after a single dose of 1% or 2% pilocarpine.17,18
Retinal Tears and Detachment
Although a very rare complication, there have been numerous case reports of peripheral retinal tears and rhegmatogenous retinal detachments occurring following topical parasympathomimetic miotic use that have been published.16,17,19-24 It is still debated whether these retinal complications were a result of a drug-induced side effect or simply coincidental.19 Although no definitive proof exists, the number of well-documented cases may suggest a cause-and-effect association.19
To date, there have been at least 100 cases of possible miotic-related retinal detachments reported in the literature attributed to various parasympathomimetic agents, some strong acting medications such as diisopropyl fluorophosphate and physostigmine, and weaker agents, including even 1% pilocarpine.17,20 The majority of retinal detachments reported occurred in susceptible eyes with risk factors for detachment, including myopia, aphakia, and peripheral retinal pathology including lattice, and/or a history of retinal detachment in the fellow eye.16,19-22 In most cases of detachment, miotics were prescribed for glaucoma, but several cases were related to a single use of a miotic to reverse pupillary mydriasis.19-22
Lemcke et al published 16 cases of retinal detachments occurring within 2 months of starting miotic therapy from a series of 1,465 consecutive detachments.12,23 These detachments were associated with flap tears or small holes in the equatorial region.13,23 Similarly, Pape et al published a case series of 14 eyes with retinal detachment that occurred within 2 months of beginning miotic therapy.17 They found that most of these eyes had risk factors for detachment, including myopia, aphakia, lattice degeneration in the detached eye, and/or retinal pathology in the fellow eye. However, 3 eyes (21%) had no risk factors for detachment. In his series, the average duration of miotic therapy prior to detachment was 38 days. Half of these eyes were being treated with 1% pilocarpine. Half of the eyes had myopia ranging from -1.25 to -10.75D, 5 eyes (36%) were aphakic, and 5 eyes (36%) had lattice in the detachment eye. One of the patients, a 59-year-old hyperope, returned within 48 hours with a large horseshoe tear and localized detachment after instillation of 1 drop of 1% pilocarpine at the end of a dilated eye exam, during which the peripheral retina evaluation was unremarkable.17
In 1991, a survey response study found that 77% of 430 general ophthalmologists, and 58.4% of 101 retinal specialists believed there was an association between retinal detachment and miotic use based upon their personal clinical experience.21 In addition, roughly one-third of retinal specialists felt that, if a general ophthalmologist found a predisposing peripheral retinal pathology, such as a flap tear, dialysis, lattice, or operculated break, he/she should advise prophylactic laser treatment, while about half indicated that they would prescribe a miotic and warn of possible detachment without recommending laser.21
No retinal complications were noted among the 354 treated subjects in the GEMINI 1 and GEMINI 2 phase 3 FDA trials of Vuity,25 which contains 1.25% pilocarpine hydrochloride and is dosed at 1 drop daily, compared to the typical dose of 1% pilocarpine for glaucoma, which is 1 drop 4 times per day.1,3 Recruitment into the GEMINI studies was restricted to patients with myopia of less than -4D.25
Exacerbation of Vitreofoveal Traction and Macular Hole Formation
Walker et al reported a case of acute-onset vitreofoveal traction associated with a small central scotoma from a single drop of 2% pilocarpine that was used to reverse dilation in a patient without pre-existing retinal pathology.18 Similarly, Benedict et al reported a case of transient stage 1A macular hole formation that occurred in a 69-year-old patient 1 month after initiation of 2% pilocarpine therapy 4 times a day for glaucoma.26 The impending macular hole resolved with pilocarpine discontinuation.26 In both reports, the eyes affected lacked a complete posterior vitreous detachment (PVD).18,26
Garlikov et al also reported a case of full-thickness macular hole formation in 1975 associated with posterior vitreous separation from the macula that occurred with 2% pilocarpine.17 Visual acuity declined from 20/20-1 to 20/200; however, no surgical correction was attempted.17
Vitreous Hemorrhage
The vitreous is thought to be rather firmly adherent to the retinal blood vessels, and ciliary spasm-induced anterior displacement of the vitreous may mechanically damage a blood vessel, leading to a vitreous hemorrhage. Schuman et al reported a case of a 70-year-old with pseudoexfoliation glaucoma who was started on 2% pilocarpine 4 times a day who complained of cloudy vision the day after starting therapy.27 He was found to have a mild vitreous hemorrhage localized to the anterior vitreous that resolved without surgical intervention.27
Tips to Avoid Posterior-segment Complications From Parasympathomimetic Miotic Therapy
- Screen for pre-existing retinal disease: Perform a dilated peripheral retinal assessment prior to initiating miotic therapy and consider repeating this procedure periodically during the first 2-3 months of treatment in high-risk patients. Carefully assess the status of the vitreous since the absence of a PVD likely poses a greater risk for complications. Consider macular optical coherence tomography screening on all patients prior to initiation of miotic therapy to detect subclinical vitreomacular traction (Figure 2).
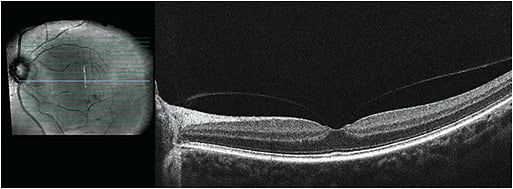
Figure 2. Vitreofoveal traction. - Avoid in patients at high risk for rhegmatogenous retinal detachment: Use with caution in individuals at high risk for detachment, such as those with lattice (especially if perivascular or radial; Figure 3), high myopia (or former high myopes who have undergone refractive surgery), aphakia, personal or family history of retinal detachment, genetic disorders such as Stickler syndrome, and patients with history of significant trauma thought to have altered the structure of the vitreous or retina.4,28 It may be worthwhile to refer patients at high risk for detachment to a retinal specialist for assessment and possible prophylactic barrier laser prior to initiating miotic therapy if it is necessary (Figure 3).19,21

Figure 3. Perivascular lattice before and after prophylactic barrier retinopexy. - Avoid in patients with vitreofoveal traction: Miotics are probably best avoided in patients with vitreofoveal or vitreomacular traction (Figure 2). Smaller zones of macular attachment likely impose a greater risk for complications since the tractional force per area increases as the size of the vitreomacular adhesion zone decreases.
- Educate patients on symptoms of retinal detachment: Educate patients (and document in the chart) started on miotic therapy about the symptoms of retinal break/detachment and advise them to return to the clinic immediately if they occur. Document that you discussed with the patient instructions for use, risks and benefits, side effects, and the symptoms that may be associated with retinal complications.
- Consider a step-wise approach to prescribing: If pharmacologic pupillary miosis is deemed desirable, consider trying an agent that inhibits the dilator (and therefore spares ciliary body contraction) first. If the effects are inadequate, then consider moving to a parasympathomimetic agent.
- Avoid unnecessary use of strong parasympathomimetics: It is probably best to refrain from using strong parasympathomimetic agents to reverse pupillary dilation after the eye exam.
Conclusion
As more parasympathomimetic miotics enter the market, it is important to keep in mind the best clinical practices that should be exercised when prescribing. It is prudent for eyecare providers to refamiliarize themselves with the potential posterior-segment side effects that could occur and minimize them whenever possible.18,29 ■
References
- Crawley L, Zamir SM, Cordeiro MF, Guo L. Clinical options for the reduction of elevated intraocular pressure. Ophthalmol Eye Dis. 2012;4:43-64.
- Wesley G, Karpecki P. A first look at therapeutics for presbyopia. 2021. Review Education Group website. October 14, 2021. Accessed April 21, 2022. https://www.revieweducationgroup.com/ce/a-first-look-at-therapeutics-for-presbyopia?fbclid=IwAR2dmBBxuJ_smQPlmY8dqgw9S2-7-2r7C6Zj_PoMDDx8ZBqURPnqGdvCuyo .
- Vuity™ package insert and prescribing information. U.S. Food and Drug Administration. Accessed April 21, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214028s000lbl.pdf .
- Fraunfelder FT, Fraunfelder FW. Drug-Induced Ocular Side Effects. 8th ed. Elsevier; 2021.
- Kato COS, Shimizu K, Kamiya K, et al. Effects of brimonidine tartrate 0.1% ophthalmic solution on the pupil, refraction, and light reflex. Sci Rep. 2018;8(1):9003.
- Asnaashari P. Managing miotics and mydriatics. Review of Optometry website. May 15, 2021. Accessed April 21, 2022. https://www.reviewofoptometry.com/article/managing-miotics-and-mydriatics.
- Aceclidine. National Center for Advancing Translational Sciences website. Accessed April 21, 2022. https://drugs.ncats.io/drug/0578K3ELIO
- Karpecki P. Solving symptoms by going off-label. Review of Optometry website. February 25, 2002. Accessed April 21, 2002. https://www.reviewofoptometry.com/article/solving-symptoms-by-going-off-label
- Skaat A, Rosman MS, Chien JL, et al. Effect of pilocarpine hydrochloride on the Schlemm canal in healthy eyes and eyes with open-angle glaucoma. JAMA Ophthalmol. 2016;134(9):976-981.
- Meyler L. Meyler’s Side Effects of Drugs: The International Encyclopedia of Adverse Drug Reactions and Interactions. 16th ed. Aronson JK, ed. Elsevier Science; 2016.
- Abramson DH, Coleman DJ, Forbes M, Franzen LA. Pilocarpine: effect on the anterior chamber and lens thickness. Arch Ophthalmol. 1972;87(6):615-620.
- Koeppl C, Findl O, Kriechbaum K, Drexler W. Comparison of pilocarpine-induced and stimulus-driven accommodation in phakic eyes. Exp Eye Res. 2005;80(6):795-800.
- Garlikov RS, Chenoweth RG. Macular hole following topical pilocarpine. Ann Ophthalmol. 1975; 7(10):1313-1316.
- Shao Y, Jiang Q, Hu D, et al. Axial elongation measured by long scan depth optical coherence tomography during pilocarpine-induced accommodation in intraocular lens-implanted eyes. Sci Rep. 2018;8(1):1981.
- Besharse JC, Dana R, Dartt DA, eds. Encyclopedia of the Eye. Elsevier/Academic Press; 2010.
- Singh M. “Miotic-induced retinal detachment”: a case report. Med J Malaysia. 1985 Jun;40(2):136-138.
- Pape LG, Forbes M. Retinal detachment and miotic therapy. Am J Ophthalmol. 1978;85(4):558-566.
- Walker JD, Alvarez MM. Vitreofoveal traction associated with the use of pilocarpine to reverse mydriasis. Eye (Lond). 2007;21(11):1430-1431.
- Salmon JF, Harpur PJ. Pilocarpine gel as a cause of retinal detachment. In: Glaucoma. 1991;13(1):28-28.
- Beasley H, Fraunfelder FT. Retinal detachments and topical ocular miotics. Ophthalmology. 1979;86(1):95-98.
- Kraushar MF, Steinberg JA. Miotics and retinal detachment: upgrading the community standard. Surv Ophthalmol. 1991;35(4):311-316.
- Puustjärvi T. Retinal detachment during glaucoma therapy. Ophthalmologica. 1985;190(1):40-44.
- Lemcke HH, Pischel DK. Retinal detachments after the use of phospholine iodide. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1966;47:157-163.
- Alpar JJ. Miotics and retinal detachment: a survey and case report. Ann Ophthalmol. 1979;11(3):395-401.
- Waring GO 4th, Price FW Jr, Wirta D, et al. Safety and efficacy of AGN-190584 in individuals with presbyopia: the GEMINI 1 phase 3 randomized clinical trial. JAMA Ophthalmol. 2022:e220059.
- Benedict WL, Shami M. Impending macular hole associated with topical pilocarpine. Am J Ophthalmol. 1992;114(6):765-766.
- Schuman JS, Hersh P, Kylstra J. Vitreous hemorrhage associated with pilocarpine. Am J Ophthalmol. 1989;108(3):333-334.
- AAO PPP Retina/Vitreous Committee; Flaxel CJ, Adelman RA, Lim JI, et al. Posterior vitreous detachment, retinal breaks, and lattice degeneration PPP 2019. Accessed April 21, 2022. https://www.aao.org/preferred-practice-pattern/posterior-vitreous-detachment-retinal-breaks-latti .
- Al-khersan H, Flynn Jr HW, Townsend JH. Retinal Detachments Associated with Topical Pilocarpine Use for Presbyopia: Pilocarpine-Associated Retinal Detachments. American Journal of Ophthalmology, 2022, https://doi.org/10.1016/j.ajo.2022.05.011 .










