AS THE FIRST AND ONLY ADJUSTABLE intraocular lens (IOL), the Light Adjustable Lens (LAL) from RxSight has redefined the cataract surgery patient journey. With traditional fixed IOLs, postoperative refractive surprises still occur despite extensive preoperative lifestyle assessments and prediction processes. The LAL overcomes these limitations by enabling optimization based on the actual refraction after surgical healing. Patients have the unique ability to trial and adjust their vision until it meets their lifestyle requirements, resulting in fewer LASIK enhancements and IOL exchanges.
As a premium IOL with the greatest likelihood of providing the best uncorrected vision at all distances, virtually all cataract surgery patients can be upgraded to the LAL. This includes patients who want the best quality of vision and postrefractive and refractive lens exchange patients. The LAL is approved for patients with preexisting astigmatism of ≥0.75D and should not be used in patients with macular disease or those who are taking systemic medication that may increase sensitivity to ultraviolet (UV) light.1

The LAL corrects in 0.25D increments of sphere and cylinder (twice as precise as standard premium IOLs) and corrects down to 0.5D of astigmatism.1 With customized blended vision, LAL patients can achieve excellent vision at all distances without the glare and halos associated with multifocal IOLs. In fact, 75% of LAL patients select blended or monovision.2
Proprietary LAL Technology
The RxSight technology, which enables postoperative adjustability, is based on the principles of photochemistry. The LAL is an UV-absorbing, photoreactive silicone IOL. When a specific pattern of UV light is delivered to the implanted LAL with the Light Delivery Device (LDD), it induces photopolymerization of shorter polymer chains called macromers in the portion of the lens matrix that is exposed. Over the next 24 hours, macromers from other areas of the lens move into the exposed volume, resulting in a precise refractive change.
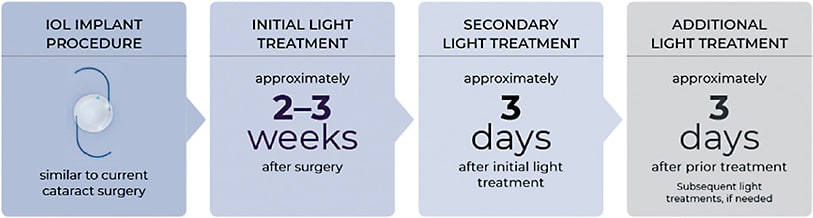
Light treatments are painless and noninvasive and take approximately 90 seconds. The initial light treatment occurs at least 17 days after surgery, with additional treatments as needed spaced 3 days apart until the patient’s desired vision is achieved. The entire lens is then polymerized with a final lock-in treatment to provide stable correction. Patients wear special UV-protective glasses when they are exposed to outside daylight until 24 hours after the lock-in step is completed, after which they are no longer required.1
Patients must have the motivation and the ability to return to the office postoperatively for multiple visits and must commit to wearing the UV protective glasses as instructed. Despite these extra steps, many patients choose the LAL because they recognize that it can provide the vision they desire with a high level of certainty.
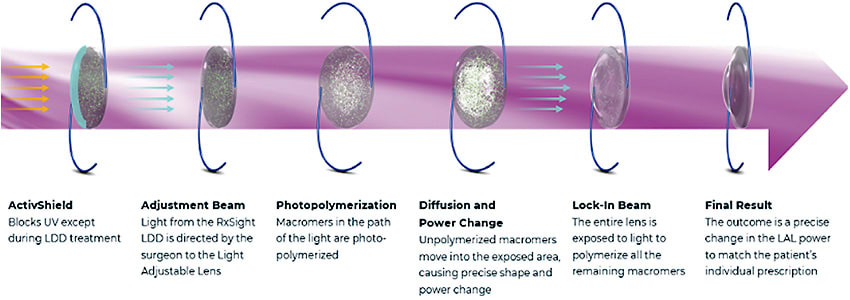
ActivShield Technology
The second-generation LAL incorporates ActivShield UV Protecter, which blocks natural UV light—except during treatment with the LDD—to protect the LAL against unintended refractive changes. Before ActivShield technology, UV-protective glasses were required to be worn at all times until the final lock-in treatment. Now, they are only needed when patients are exposed to outside daylight, reducing potential compliance concerns for surgeons and their patients.
Clinical Data: LAL Patients Twice as Likely to See 20/20
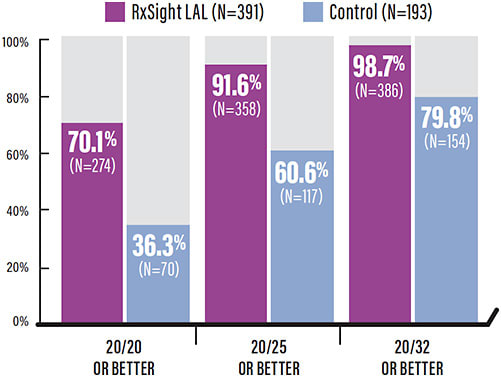
FDA approval was based on results of a US randomized, controlled, prospective, multicenter study comparing the LAL to a commercially available monofocal lens (control). Efficacy was evaluated at 6 months in 391 LAL eyes and 193 control eyes. The results showed that the percentages of LAL eyes within 0.50D of sphere and 0.50D of cylinder were 92% and 82%, respectively, similar to the refractive accuracy seen in recent LASIK studies. LAL eyes achieved 20/20 vision or better at approximately twice the rate of patients receiving a monofocal lens (70.1% vs 36.3%, respectively).3
The LAL study also demonstrated that fewer LAL patients had poor results vs control, defined as eyes outside of 1.0D of sphere (0.5% vs 3.1%) or cylinder (1.5% vs 25.4%) and eyes 20/40 or worse (1.3% vs 20.2%).3
In a recent prospective, commercial study, the visual outcomes of 121 patients bilaterally implanted with the LAL with ActivShield were evaluated at 13 practices in the United States. Monocular and binocular uncorrected and bifocal-corrected distance visual acuity under photopic conditions and subjective manifest refraction were assessed approximately 2 months after the second eye implantation. The results demonstrated that LAL eyes were twice as likely to achieve 20/20 vision or better and 10 times less likely to see 20/40 or worse compared to standard premium IOLs.2
The LAL Procedure
The preoperative assessment and LAL procedure are straightforward and no different than for standard cataract surgery. Patients should have a pristine ocular surface and eyes that are able to dilate to a pupil diameter >6.5 mm so that the edge of the LAL can be visualized during postoperative light treatments.
Postoperative light treatments are performed on both eyes at the same time. The initial light treatment is typically delivered 2 to 3 weeks after surgery, and additional light treatments, if necessary, are performed at least 3 days after the previous treatment. Patients receive up to 3 light treatments for refractive adjustment and then a final lock-in treatment to prevent any further refractive change of the LAL.
It is important to manage patient expectations before and after the light treatments. After the initial surgery and before light treatments, patients will see well enough to perform activities of daily living, with vision similar to what their final vision would be with a nonadjustable IOL. After the first LDD treatment, patients will notice the greatest difference in their vision. With the second and third treatments, you will be fine-tuning to achieve the high-quality, customized vision for which your patients paid a premium price.
Integrating the LAL Into Your Practice
The process of LAL integration into the practice is not as difficult as it appears from the onset. After performing cataract surgery on each eye with the LAL (typically 1 week apart), the process of adjustments and lock-in is all planned ahead of time with the surgery coordinator and can be altered based on the healing characteristics specific to the patient. In some states, the OD for the practice can perform the LDD treatments, or if the practice has a fellowship program like mine, the fellow can be certified to perform the LDD treatments.
The most important part of the postoperative LDD adjustments is making sure you obtain accurate refractions, and I recommend at least 2 good refractions be performed prior to the LDD treatment-decision process. Do not rely on autorefraction devices at this stage of the process. The target I aim for in most patients is a blended customized vision, shooting for plano in the dominant eye and typically -0.75D sphere in the nondominant eye with just the cataract surgery.
| MORE PATIENTS WITH EXCELLENT RESULTS | COMPARISON IOL | LAL2 (N=121 DISTANCE EYES) |
|---|---|---|
| Eyes within 0.50D of sphere | 74%4 | 93.4% |
| Eyes within 0.50D of cylinder | 62-64%5,6 | 90.6% |
| Eyes 20/20 or better | 38-41%5,6 | 80.2% |
| FEWER PATIENTS WITH POOR RESULTS | COMPARISON IOL | LAL2 (N=121 DISTANCE EYES) |
|---|---|---|
| Eyes outside 1.0 D of sphere | 6%4 | 0.8% |
| Eyes outside 1.0 D of cylinder | 10-11%5,6 | 1.3% |
| Eyes 20/40 or worse | 11-17%5,6 | 0.8% |
Obviously, if the patient has any astigmatism that needs correction, it will not be corrected until the first LDD adjustment, and patient expectations should be set preoperatively. If a patient wants more near vision, I typically only add a maximum of -0.50D sphere in the adjustment, as the LAL has some extended depth of field effect, eliminating the need for a full monovision target. The LAL definitely delivers on all fronts in terms of adjustability, invisibility with the built-in ActivShield UV protection, customizability, and profitability for the practice, as stated below.
Practice Economics With the LAL
The results of a real-world economic impact study demonstrate how the LAL is enabling practices to build their premium IOL business by converting conventional and premium procedures into the premium LAL.7
This study found that approximately one-third of LAL procedures are done in patients who would have otherwise received a monofocal IOL and therefore not generated any additional practice revenue beyond the standard reimbursement. Another third come from patients who were upgrading to the LAL from lower-priced competing astigmatism-correcting IOL procedures, which typically leads to a 2-fold increase in revenue.7
The remaining third are patients who otherwise would have received multifocal IOLs. These are patients who desire excellent vision at all distances but might be concerned about the side effects of glare, halo, and loss of contrast. Although this latter group has the smallest pricing differential compared to the LAL, the great visual outcomes that these patients experience are key to driving more future referrals to the practice.7
On average, each LAL case increased net revenue by more than $1,600 at these mostly early adopter practices. The study also looked at practices with lower national average pricing, finding that each LAL case increased net revenue by more than $1,200. In summary, the combination of higher pricing, an expanded premium patient pool, and enhanced referrals, drives practice revenue and profit gains for practices that offer the LAL.7 ■
References
- RxSight, Inc. Light Adjustable Lens (LAL) and Light Delivery Device (LDD) Professional Use Information.
- RxSight PMCS-002 Clinical Outcomes of Patients Bilaterally Implanted with LAL.
- RxSight PMA P160055: FDA Summary of Safety and Effectiveness Data. 2017.
- Lundstrom M, Dickman M, Henry Y, et al. Changing practice patterns in European cataract surgery as reflected in the European Registry of Quality Outcomes for Cataract and Refractive Surgery 2008 to 2017. J Cataract Refract Surg. 2021;47(3):373-378.
- Tecnis Toric PMA P980040/S039: FDA Summary of Safety and Effectiveness Data. 2013.
- AcrySof Toric P930014/S15: FDA Summary of Safety and Effectiveness Data. 2011.
- RxSight Economic Impact Study by Haffey & Company.









