LASER VISION CORRECTION has been a mainstay in the refractive surgery world since the mid-1990s. LASIK specifically is a household name; it is tried and true. Unfortunately, laser vision correction is not an option for everyone. The EVO implantable collamer lens (ICL) (Staar Surgical) has opened the door for patients who may have previously been less than ideal candidates for corneal refractive surgery. EVO has an outstanding safety profile and provides excellent visual quality that rivals (and arguably outperforms) modern corneal refractive techniques. Although not available yet in the United States, the extended depth of focus EVO Viva has received promising feedback for presbyopic myopes in Europe.
Phakic Intraocular Lenses: the Paradigm Shift in Refractive Surgery
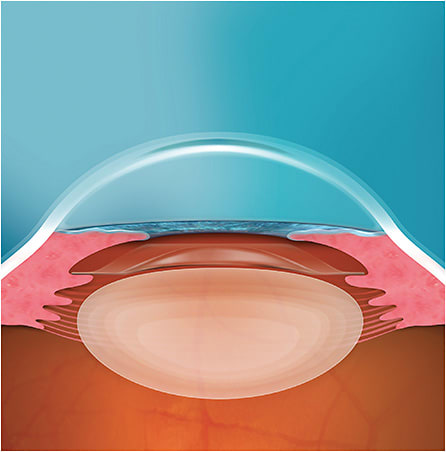
LASIK is a dose-dependent procedure; therefore, patients with higher degrees of myopia or thinner corneas are not considered ideal candidates due to the degree of stromal ablation necessary to treat higher refractive errors. Additionally, postoperative dry eye and induced glare/halos can become very real problems for post-LASIK patients, especially at the higher end of the myopic spectrum. How do we get around a thin/irregular cornea? Simple—by completely avoiding it. By leaving the epithelium intact, dryness after surgery is significantly reduced, as are postoperative dysphotopsias. Phakic intraocular lenses (IOLs) have been around since the mid-1950s. The technology at the time was far from perfect and came with high complication rates. By the mid-2000s, the ICL gained FDA approval. The ICL, unlike early phakic IOLs, is implanted into the ciliary sulcus anterior to the crystalline lens. Earlier-generation phakic IOLs were implanted into the anterior-chamber angle or were anchored to the iris. These lenses came with a very high incidence of corneal decompensation and iris-related irregularities. Positioning the ICL posterior to the iris preserves the delicate corneal endothelium while providing crystal clear optics. Corneal refractive surgery naturally alters corneal architecture, making refractive precision more difficult during subsequent cataract surgery. Patients are comforted by its being easily removable, meaning there is no influence on refractive targeting during cataract surgery later in life, ultimately meaning all premium IOLs are on the table as options for these patients.
A Hole in One!
Safety profile was a real concern for many surgeons with the Visian ICL, mainly due to the risk of pupillary block or premature cataract formation. While the Visian showed excellent optical quality, the understandable safety concerns led to a reluctance to adopt in many refractive surgery clinics. The Visian ICL also required a peripheral iridotomy to preserve normal aqueous flow. In our clinic, these iridotomies were performed with a laser 1–2 weeks before surgery. In 2022, the EVO ICL was introduced, which has all but put many of the safety concerns to rest. The main difference between the EVO and its predecessor is the addition of a central 360-µm aquaport that allows aqueous exchange between the posterior and anterior chambers. The port eliminates the need for iridotomy, thereby streamlining the entire process and sparing the patient from a potentially uncomfortable presurgical procedure. Most importantly, since the introduction of EVO, complication rates have plummeted. The lens comes in four diameters: 12.1, 12.6, 13.2, and 13.7 mm. Choosing the correct size is crucial for safety. If it is too large, the vault over the crystalline lens is too great and could result in angle closure. If it is too small, there is a risk of contact with the anterior capsule, possibly igniting early cataract development. The central port significantly minimizes these risks by keeping proper flow from the ciliary body to the trabecular meshwork, preserving normal ocular physiology. In fact, in the FDA clinical trial, among 629 eyes, there was no incidence of pupillary block, cataract development, or pigment dispersion after six months.1 This outcome is consistent with what has been published internationally with much longer follow-up times.2

The Nodal Point
The EVO ICL has a spherical equivalent range of -3.00 to -20.00 D, with an additional -4.00 D cylinder at the corneal plane. The nodal point of the human eye sits near the anterior plane of the crystalline lens, so correcting high degrees of myopia as close to the nodal point as possible is clearly advantageous because of the reduction in retinal minification compared to correcting myopia on the corneal plane or, further yet, on the spectacle plane. In the FDA study, 98.5% of patients had corrected distance visual acuity (CDVA) equal to or better than preoperative visual acuity, while 52.3% gained lines of CDVA.2,3 Because of its placement near the nodal point, the EVO ICL has a very forgiving optical profile. Because of this, refractive accuracy is consistent across the entire dioptric profile, with 90.5% of patients being within 0.5 D of target. It has been shown in previous studies that, with the earlier version of the ICL, there were fewer induced higher-order aberrations compared to wavefront-guided LASIK. In a more recent study looking specifically at EVO vs small incision lenticule extraction (SMILE), retinal magnification was 1.0 in the EVO group, compared to 0.97 in the SMILE group. Overall, the EVO group showed a slightly superior postop visual quality compared to the SMILE group, suggesting that the introduction of the central port has little to no visual effect.4
The Presbyopic Patient
The EVO ICL is indicated in the United States for patients ages 21–45. There are of course off-label considerations for patients older than 45 who may not be ready for cataract surgery or refractive lens exchange. EVO, like corneal refractive surgery, leaves the natural accommodative system intact, meaning reading glasses will still be a part of normal life in presbyopic patients. In our practice, we often will target the dominant eye for plano, while targeting the nondominant eye slightly myopic (typically between -0.50 and -1.00 D) in these patients. Doing so retains excellent binocular distance visual acuity (DVA) while preserving depth perception and depth of focus. Recent studies of early presbyopic EVO ICL patients have shown retained stereopsis with a good range of near vision using this technique, confirming monovision, or “blended vision” as we like to call it, as a solid surgical strategy for this demographic.5
On the horizon, the EVO Viva is a presbyopia-correcting ICL that has recently been introduced in Europe. The Viva is indicated for the elimination or reduction of myopia in patients with -0.50 to -20.0 D of myopia on the spectacle plane with an anterior chamber depth of at least 2.8 mm. First introduced in 2020, it includes an aspheric optic and is indicated for patients between 21 and 60 years old. Interestingly, Viva is an option for both phakic and pseudophakic patients, meaning there is the opportunity for near correction for patients who have already undergone cataract surgery and may not be candidates for lens exchange. The Viva maintains the central port, allowing for a matched safety profile to the monofocal EVO ICL. The asphericity of the lens aims to increase spherical aberration, extending depth of focus by about 2.0 D.6 It has been reported that the EVO Viva increased depth of focus without compromising best-corrected DVA or contrast sensitivity, while maintaining the refractive accuracy and safety profile of its monofocal counterpart. In one study, it was reported that the necessary add power for presbyopic patients changed from +1.31 ± 0.74 D preoperatively to +0.44 ± 0.58 D after surgery with no significant DVA compromise.7 Unfortunately, the Viva is only available in Europe with no timetable set for release in the United States.
While corneal refractive surgery continues to expand with new techniques and procedures, the ICL has opened the door for a subset of patients who previously were noncandidates for refractive procedures. The introduction of the EVO ICL last year has dramatically improved safety measures while allowing for pristine optical outcomes. The EVO Viva, although not available in the United States, seems to be a solid option for presbyopic myopes and will certainly be something to look forward to in the coming years. ■
References
- Packer M. Evaluation of the EVO/EVO+ sphere and toric Visian ICL: six-month results from the United States Food and Drug Administration clinical trial. Clin Ophthalmol. 2022;16:1541-1553.
- Packer M. The Implantable Collamer Lens with a central port: review of the literature. Clin Ophthalmol. 2018;12:2427-2438.
- Packer M. United States multicenter clinical trial of a posterior chamber phakic implantable lens with a central port for myopia or myopic astigmatism. Paper presented at: Annual meeting of the American Society of Cataract and Refractive Surgery; Washington, DC; April 24, 2022.
- Qin Q, Bao L, Yang L, He Z, Huang Z. Comparison of visual quality after EVO-ICL implantation and SMILE to select the appropriate surgical method for high myopia. BMC Ophthalmol. 2019;19(1):21.
- Kamiya K, Takahashi M, Takahashi N, Shoji N, Shimizu K. Monovision by implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for early presbyopia. Sci Rep. 2017;7(1):11302.
- Packer M, Alfonso JF, Aramberri J, Elies D, Fernandez J, Mertens E. Performance and safety of the extended depth of focus implantable collamer® lens (EDOF ICL) in phakic subjects with presbyopia. Clin Ophthalmol. 2020;14:2717-2730.
- Alfonso JF, Fernandez-Vega-Cueto L, Lisa C, Alfonso-Bartolozzi B, Palacios A, Madrid-Costa D. Clinical and aberrometric outcomes of a new implantable collamer lens for myopia and presbyopia correction in phakic patients. J Refract Surg. 2023;39(9):589-596.








