SURGICAL PRESBYOPIA CORRECTION options continue to evolve and expand. There is a new presbyopia-correcting intraocular lens (IOL) choice now available in the United States for patients and surgeons. The FDA approved in July 2022 the ClearView 3 from Lenstec, Inc., which is the first refractive, rotationally asymmetric, multifocal IOL to become commercially available in the United States.
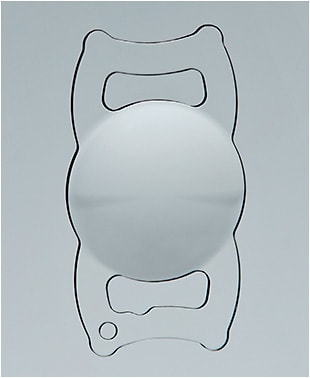
The ClearView 3 posterior-chamber IOL is an aspheric, single piece IOL (Figure 1). The optic and haptics are both acrylic (hydroxyethylmethacrylate—26% water). The IOL has a 5.75mm optic with closed loop/modified-plate haptics. This haptic design provides good stability and helps to avoid any IOL tilt, which can negatively impact the performance of this type of design.1 Different from symmetric multifocal IOL designs, which feature concentric rings providing different focal points, the ClearView 3 optic has 2 distinct zones—a distinct distance zone and a distinct near zone with a smooth transition area between them. The near zone has a +3.00D on the IOL plane, equating to an approximately 2.4D add on the spectacle plane. The index of refraction of the IOL is 1.456, and the recommended A constant is 118.0. The lens is available in dioptric range from 15.0D to 30.0D. It is manufactured in 0.25D steps in IOL powers from 15.0D to 25.0D to allow for greater ability to achieve the surgical refractive target.
Study Results
The FDA study of the ClearView 3 lens, as well as others, showed good patient satisfaction and visual outcomes after implantation.2-4 The FDA Investigational Device Exemption study of the ClearView 3 lens was a prospective, subject-masked, randomized, 2-arm, parallel group study. It included 340 subjects bilaterally implanted with the ClearView 3 multifocal IOL and 170 bilaterally implanted with a monofocal control IOL across 18 clinical sites.5 The patients were followed up at 1 day, 1 to 2 weeks, 1 to 2 months, 4 to 6 months, and 1 year. The ClearView 3 provided significantly better uncorrected near visual acuity and distance corrected near visual acuity than the control lens in the clinical trial. At 1 year postoperatively, 99% of ClearView 3 patients had better than 20/40 uncorrected distance visual acuity (UCDVA), 96.2% had better than 20/32 UCDVA, and 83.4% had better than 20/25 UCDVA or better, compared to only 26.7%, 5.6%, and 2.5% of patients, respectively, with the control lens.5 The study also demonstrated that the ClearView 3 multifocal IOL had comparable UCDVA and best corrected distance visual acuity (BCDVA) to that of the control IOL, with 100% of patients with the study lens and control lens with BCDVA of 20/32 or better and 97.9% of study patients with BCDVA of 20/25 or better, compared to 100% of control patients.5
Although the lens has distinct distance and near segments, multiple studies have shown good uncorrected intermediate visual acuity (UCIVA).2-4 In the FDA clinical trial, the ClearView 3 IOL demonstrated improved UCIVA and distance corrected intermediate visual acuity compared to the control IOL. At 1 year postoperatively, 97.1% of patients with bilateral ClearView 3 IOL had UCIVA of 20/32 or better compared to 64.6% of control patients; 83.4% of the ClearView 3 patients had 20/25 or better UCIVA compared to 27.3% of control patients.5 This intermediate visual acuity is considered to come either from the gradual transition zone between the 2 refractive areas of the IOL or from some induction of aberration from the IOL design providing a larger depth of focus.2
Patient Satisfaction
At the final FDA study postoperative examination, the patients in the study were asked to rate on a scale of 1 to 5 how often they needed spectacle correction at distance, intermediate, and near, with a grade of 1 equaling never. Patients rated the need for distance correction at 1.26, intermediate correction at 1.27, and near correction at 1.33, showing very good patient satisfaction with the vision provided by the lens in the clinical trial.5 The patient satisfaction reported in this study was very consistent with the satisfaction reported in multiple other studies of the ClearView 3 IOL.2-4
Lens Placement and Patient Selection
The manufacturer’s recommendation is to implant the lens with the near segment oriented inferonasally in both eyes. The bifocal design of the lens does allow the surgeon to orient the lens in an alternative rotational position. There have been several studies demonstrating that different placements of the lens are well tolerated.6,7 This unique feature of the lens may expand the number of multifocal IOL candidates. A patient with a large angle kappa may be excluded from using some multifocal IOLs due to concerns about poor effects or reduced visual quality of vision from an implant not centered on the visual axis. With the ClearView 3 IOL, a surgeon has the ability to evaluate the placement, and he or she can adjust the orientation of the distance segment during surgery, utilizing the first Purkinje image as a reference. An early study showed potentially improved vision at an intermediate distance with a customized implantation approach vs standardized inferonasal placement as well.6 Additionally, rotation of the IOL to move the distance segment into a more optimal position for an individual patient, could provide a surgical solution for a patient with visual concerns from the multifocal nature of the IOL, prior to considering explant to a monofocal IOL.
In our experience, good candidates for the ClearView 3 IOL include patients with generally healthy corneal and retinal health and, as with all multifocal IOL patients, a reasonable level of visual expectations from the technology. The IOL is not manufactured in toric powers, so patients requiring a toric lens are not ideal candidates for this technology, nor are patients needing IOL power outside of the manufactured range.
Case Study
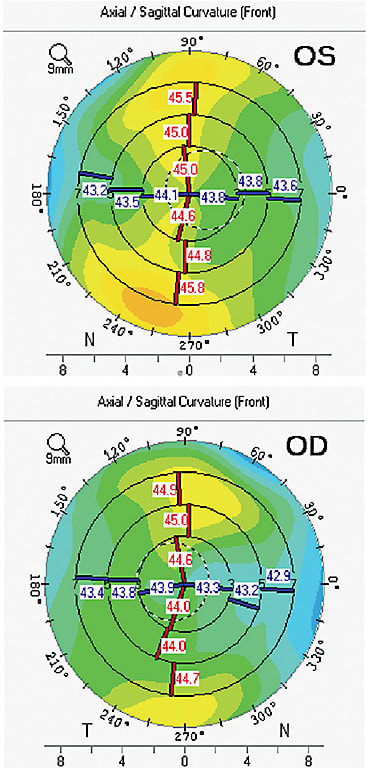
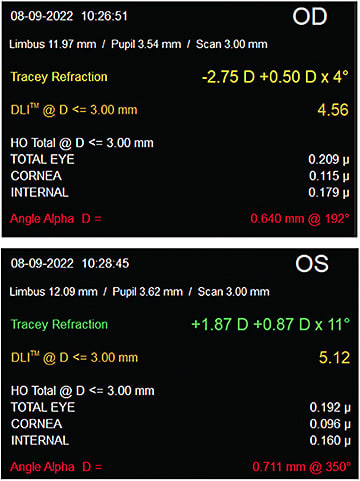
The ClearView 3 lens was the lens of choice for a recent patient at our clinic. A 77-year-old woman with visually significant cataracts presented to us desiring reduced dependence on glasses at all distances. She had normal corneal and retinal health on dilated examination. Her corneal topography was normal and measured 0.53D OD and 0.11D of corneal cylinder (Figure 2). During preoperative testing her angle alpha measured 0.711 OD and 0.640 OS with iTrace imaging (Tracey Technologies; Figure 3). Given this finding, a multifocal implant with a concentric ring design may have resulted in a greater likelihood of visual challenges postoperatively. Prior to the ClearView 3 approval, this patient may have been encouraged to consider a monofocal or extended depth of focus type of lens vs a multifocal IOL. The ability to control the placement of the distance vision segment of the ClearView 3 IOL provided confidence to still offer a multifocal option.
The patient chose to proceed with cataract surgery with the ClearView 3 IOL. A 23.5D implant was successfully implanted in both eyes with the distance vision segment oriented over the first Purkinje image in both eyes. At 1 month postoperatively, the patient was very happy with her vision and using minimal glasses. Her uncorrected distance acuity measured 20/20 OD, 20/20 OS, and 20/20 OU. Her UCIVA measured 20/12.5 OD, 20/16 OS, and 20/16 OU, and her UCNVA measured J1 OD, J1+ OS, and J1+ OU.
Happier Patients
The FDA approval of the ClearView 3 IOL provides another tool for surgeons to use to help patients improve their visual function and lifestyle after cataract surgery. More options for surgical presbyopia correction are enabling a customized approach to refractive cataract surgery. This ability to match technology to the appropriate patient continues to help surgeons and patients achieve great visual results. ■
References
- Montes-Mico R, Lopez-Gil N, Perez-Vives C, Bonaque S, Ferrer Balsco T. In vitro optical performance of nonrotational symmetric and refractive-diffractive aspheric multifocal intraocular lenses: impact of tilt and decentration. J Cataract Refract Surg. 2012;38(9):1657-1663.
- Venter JA, Barclay D, Pelouskova M, Bull CE. Initial Experience with a new refractive rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2014;30(11):770-773
- McNeely R, Pazo E, Spence A, et al. Visual outcomes and patient satisfaction 3 and 12 months after implantation of a refractive rotationally asymmetric multifocal intraocular lens. J Cataract Refract Surgery. 2017;43(9):633-638.
- Wang X, Tu H, Wang Y. Comparative analysis of visual performance and optical quality with a rotationally asymmetric multifocal intraocular lens and an apodized diffractive multifocal intraocular lens. J Ophthalmol. 2020;2020:7923045.
- Lenstec, Inc. Data on file.
- Liu Y, Gao Y, Liu R, et al. Influence of angle kappa-customized implantation of rotationally asymmetric multifocal intraocular lens on visual quality and patient satisfaction. Acta Ophthalmol. 2020:98(6):e734-e742.
- Song IS, Yoon SY, Kim JY, Kim JH, Tchah H. Influence of near-segment positioning in a rotationally asymmetric multifocal intraocular lens. J Refract Surg. 2016;32(4):238-243.










