
Introduction
MULTIFOCAL OPTICS ARE PART OF OUR NATURAL OPTICAL system and can be a beautiful part of our postoperative cataract surgery visual life, if we doctors do our best to close the loop on a comprehensive multifocal implant surgery experience. Having worked with multiple modern day multifocal implants, and witnessed high rates of patient satisfaction with them all, has taught me there is a common denominator to achieving success with them. That common denominator can be summarized in one word, optimization.
I have found it interesting that doctors will do their very best to refract a patient to plano and prescribe the best glasses prescription for patient satisfaction after traditional monofocal implant cataract vision but leave low levels of untreated, visually significant, refractive error in patients with multifocal implants seeking quality vision without glasses. The path to visual joy is simply not that different with a multifocal patient compared to a monofocal patient. Both types of patients can be quite happy if their eye health is great from front to back, and their tear film, implant centration, posterior capsule, and refractive error are treated to the best of our ability. In this article, I review how to optimize the patient’s optical system, along with how to set proper expectations (for the doctor and the patient) regarding the multifocal implant journey.
The 2 Major Optimization Phases of the Multifocal Implant
1-year Journey
Quality patient selection and education include setting up the operating surgeon and his or her team’s expectations, along with the patient’s expectations. Industry has blessed us with amazing implant technologies, and we must continue to progress in our understanding of how to optimally deliver them to our patients. Multifocal implants are delivering some of the highest implant vision patient satisfaction ever, and wow, can it be a boost to the enjoyment of practicing vision correction surgery when you are practicing on the cutting edge, and you, your team, and your community are enjoying the benefits of doing so.
I like patients to understand there are 2 basic time periods in the multifocal implant journey: the period of optimization of their vision and the time during which their brain optimizes their new optical system. The time of vision optimization includes any preoperative preparation (such as tear film treatment), the lens replacement surgery itself, and the 4 to 6 months after surgery of working to achieve a plano or near plano refraction, along with uncorrected crisp 20/20 vision in each eye alone. The second 4 to 6 months is the brain optimizing our visual adaptation to simultaneous vision (seeing distance, computers, and cell phones all at the same time), or what we call neuroadaptation.
Therefore, I like patients to understand that we are embarking on a 1-year journey, and if they remain patient during that 1-year journey, at the end they will have some of the world’s most advanced optics in their eyes and be able to enjoy the reading range of people in their 30s for the rest of their lives. This is a powerful value proposition to patients who value spectacle independence.
Even though it is not talked about as much, I also believe this is a powerful value proposition for patients who do not mind wearing glasses but who do not want to have the distortions and increased chance of falling risk that accompanies them.1 Lord and colleagues concluded that multifocal glasses impair depth perception and edge-contrast sensitivity at critical distances for detecting obstacles in the environment.1 As a result, wearers were more than twice as likely to fall compared to elderly people not wearing bifocals, trifocals, or progressives. The fact that older people may benefit from wearing single-vision glasses or no glasses when negotiating stairs and in unfamiliar settings outside the home is an important consideration in our patient education. There are patients who do not mind wearing glasses and love the idea of single-vision glasses after cataract surgery.
Don’t Worry: The Reading Halo Is for Sure
In general, multifocal implants will dedicate a majority of the visual light energy to distance and the minority to near and intermediate. The exact amount varies with different implants, but in general, you can think of most of the light energy being dedicated to distance and the minority dedicated to computer and cell phone distance. I tell patients, “The part of the implant that gives you the reading range of someone in their 30s is the same part of the implant that causes halos at a distance around lights at night. Don’t worry when you see them; expect them. They get better with time, and my work is to fine tune your vision. One of the reasons these implants were FDA approved is because they get better with time and are typically not bothersome at 1 year.” I like to say 1 year because it takes time to optimize the vision to a crisp 20/20 in each eye alone in the first 6 months whether it is tear film, refractive error, or yttrium aluminum garnet (YAG) laser capsulotomy that is needed, and then neural adaptation occurs for the next 3-6 months.
Dysphotopsias result from light that is off axis. A positive dysphotopsia after cataract surgery is an artifact or the presence of extra light, often described as glare, starbursts, or halos. This contrasts with negative dysphotopsias, manifesting as the absence of light on a portion of the retina and described as a dark, temporal, arcing shadow.
The pattern and amount of light energy that is off axis creates a characteristic and unique shape and intensity of the dysphotopsia. In general, there are 3 distinct types of dysphotopsias or distortions of a point source of light: glare, halo, and starburst (Figure 1A). Glare and starbursts generally result from posterior capsular opacification (PCO), refractive error (eg, astigmatism), optical aberrations, and/or problems with the tear film or ocular surface. Halos are the characteristic dysphotopsia caused by multifocal intraocular lenses (IOLs).

While companies have optimized trifocal optics beautifully, we need to keep up with our job and understand how to minimize aberrations by understanding and treating our patients, as well as coaching them on what to expect. Understanding that, when patients are describing glare, halo, and/or starburst, they are giving you a clue as to the source or sources. The combination of dysphotopsias can be very disconcerting to the patient and doctor, and understanding that we can treat the halo and starburst sources to optimize the neural adaptation to the multifocal halo is very important (Figure 1B). It is our job to treat what we can treat and educate on what is normal (for instance, implant-related halo) and how it typically greatly improves with time through the process of neuroadaptation.
Doctors Optimize the Patient’s Eyes So Their Brain Neuroadaptation Can Optimize Their Vision
Neuroadaptation is a very important time during which our brain adapts to and optimizes the patient’s new optical system.2 For it to occur optimally, the surgeon must follow a methodical process to completion for every multifocal patient.
For the uninformed patient, positive dysphotopsias (glare, halo, and starburst) can be a common cause of dissatisfaction after multifocal implant cataract surgery.3,4 They are also considered a leading cause of IOL explantation.5 It is important for surgeons who are implanting multifocal implants to continually remember that patient satisfaction can be very high if we play our role in the process. If patients are educated well to expect glare and halo with healing and other issues we minimize (such as refractive error, tear film, PCO) and to expect halo from the reading part of the implant that gets better with time, their satisfaction can be very high. For most patients, dysphotopsias improve over time, and it is felt that neuroadaptation plays a central role in this improvement. Although the phenomenon of neuroadaptation is not perfectly understood, its contribution to improving image quality over time is well recognized and respected. It is key that we optimize the patient’s image through quality surgery and postoperative care for optimal neuroadaptation to occur.
Optimizing Image Quality
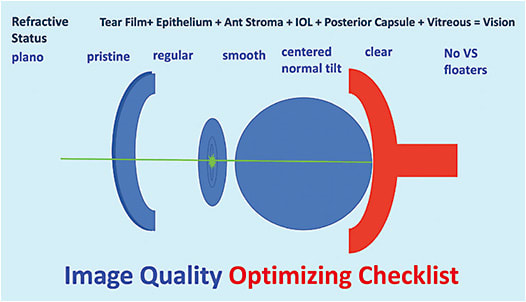
Surgical steps to optimize image quality are to center the implant on the subject-fixated coaxially sighted corneal light reflex.6 This is basically the first Purkinje image with the patient fixation. I like to also center the capsulotomy on the same image and make it 5.0mm to 5.2mm in diameter, so it overlaps the optic, and then with capsular contraction, any potential tilt or decentration of the implant is minimized (Figure 2).7,8 I also consider it very important to polish the posterior capsule through which light is traveling so that I don’t have a hazy capsule confusing things in the early postoperative period. Therefore, I like to protect the posterior capsule using a polymer tip on the irrigation/aspiration handpiece and not a metal tip to polish. I like to call this process “posterior-capsule pristining.”
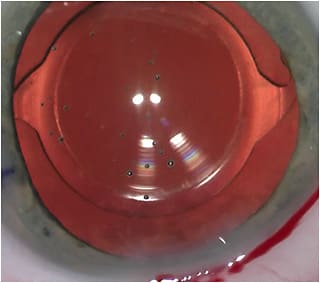
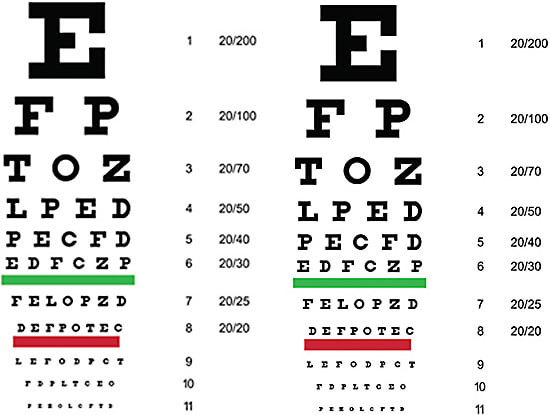
The first step in optimizing image quality postoperatively is understanding how to assess it. History is very important. If a patient says his or her image is blurred, even if he or she reads your 20/20 line, we must listen to the patient and help him or her to achieve the image being sought. If your technician writes that the vision is 20/20, he or she must also document that it is “not clear” if that is how the patient feels (Figure 3).
If the patient’s best corrected image quality, what I like to call BCIQ, is reduced, a very helpful test is a gas permeable contact lens over refraction. If the manifest refraction is not crisp and the gas permeable contact lens over refraction is crisp, we know that the patient’s issue is corneal surface related, such as tear film or epithelial issue that needs to be treated. I think about the eye from front to back to make sure all sources of blur are accounted for as we journey toward best image quality so that optimal neuroadaptation can occur (Figures 4 and 5). Before an implant exchange is considered, it is helpful to have addressed all of these potential image quality–reducing issues.
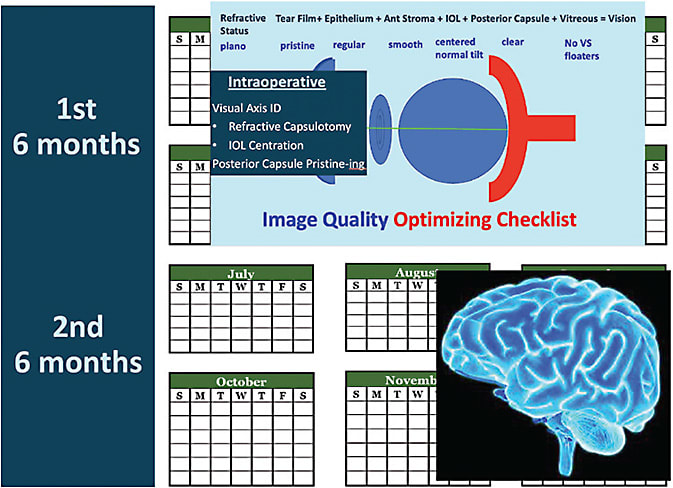
Conclusion
If you follow these steps to optimize the image quality in the first 4 to 6 months to allow for optimal neuroadaptation in the second 4 to 6 months, your patients who desire multifocality will have a high rate of patient satisfaction. Setting your and your patient’s expectations through quality preoperative education and postoperative care will take your satisfaction with delivering multifocality to another level in your practice. ■
References
- Lord SR, Dayhew J, Howland A. Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc. 2002;50(11):1760-1766.
- Rosa AM, Miranda ÂC, Patrício MM, et al. Functional magnetic resonance imaging to assess neuroadaptation to multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(10):1287-1296.
- Rosen E, Alio JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016; 42(2):310-328.
- Braga-Mele R, Chang D, Dewey S, et al; ASCRS Cataract Clinical Committee. Multifocal intraocular lenses: Relative indications and contraindications for implantation. J Cataract Refract Surg. 2014(2); 40:313-322.
- Mamalis N, Brubaker J, Davis D, Espandar L, Werner L. Complications of foldable intraocular lenses requiring explantation or secondary intervention 2007 survey update. J Cataract Refract Surg. 2008;34(9):1584-1591.
- Chang DH, Waring GO 4th. The subject-fixated coaxially sighted corneal light reflex: a clinical marker for centration of refractive treatments and devices. Am J Ophthalmol. 2014;158(5):863-874.
- Thompson V. Streamlined method for anchoring cataract surgery and intraocular lens centration on the patient’s visual axis. J Cataract Refract Surg. 2018;44(5):528-533.
- Thompson V, Holladay J, Sretavan D. Use of P1-P4 Purkinje reflections as a surrogate sign for intraoperative patient fixation. J Cataract Refract Surg. 2021;47(12):e60-e65.









