Lenz therapeutics released topline data from its CLARITY phase 3 clinical trials of LNZ100 for treatment of presbyopia, which met all primary endpoints and showed a strong safety profile. LNZ100 demonstrated rapid onset and 10-hour duration with 71%, 71%, and 40% of participants in its vehicle-controlled CLARITY 2 efficacy trial achieving a ≥3-line improvement at 0.5, 3, and 10 hours, respectively.1 The company is targeting a mid-2024 FDA submission. These trials were based on a unique and highly pupil selective cholinergic miotic, aceclidine. Aceclidine is a new chemical entity for the United States, and it targets the pupil sphincter muscle with minimal effect on the ciliary muscle. Compared to nonselective miotics, such as pilocarpine and carbachol, aceclidine’s pupil-selective mechanism of action achieves a <2-mm pupil for expanded depth-of-focus without the myopic shift and other risks associated with unwanted stimulation of the ciliary muscle.
Three CLARITY trials were conducted simultaneously: there were 2 6-week safety and efficacy studies and a single 6-month safety study evaluating LNZ100 (1.75% aceclidine). These randomized, double-masked trials had broad inclusion criteria, which included subjects 45 to 75 years old (mean 55), refractive status of -4 D to +1 D spherical equivalent with ≤2 D of astigmatism, and pseudophakes, as well as post-LASIK presbyopes. All subjects had a baseline best-corrected distance visual acuity (BCDVA) at near of 20/50 or worse to detect the required improvements in near acuity.
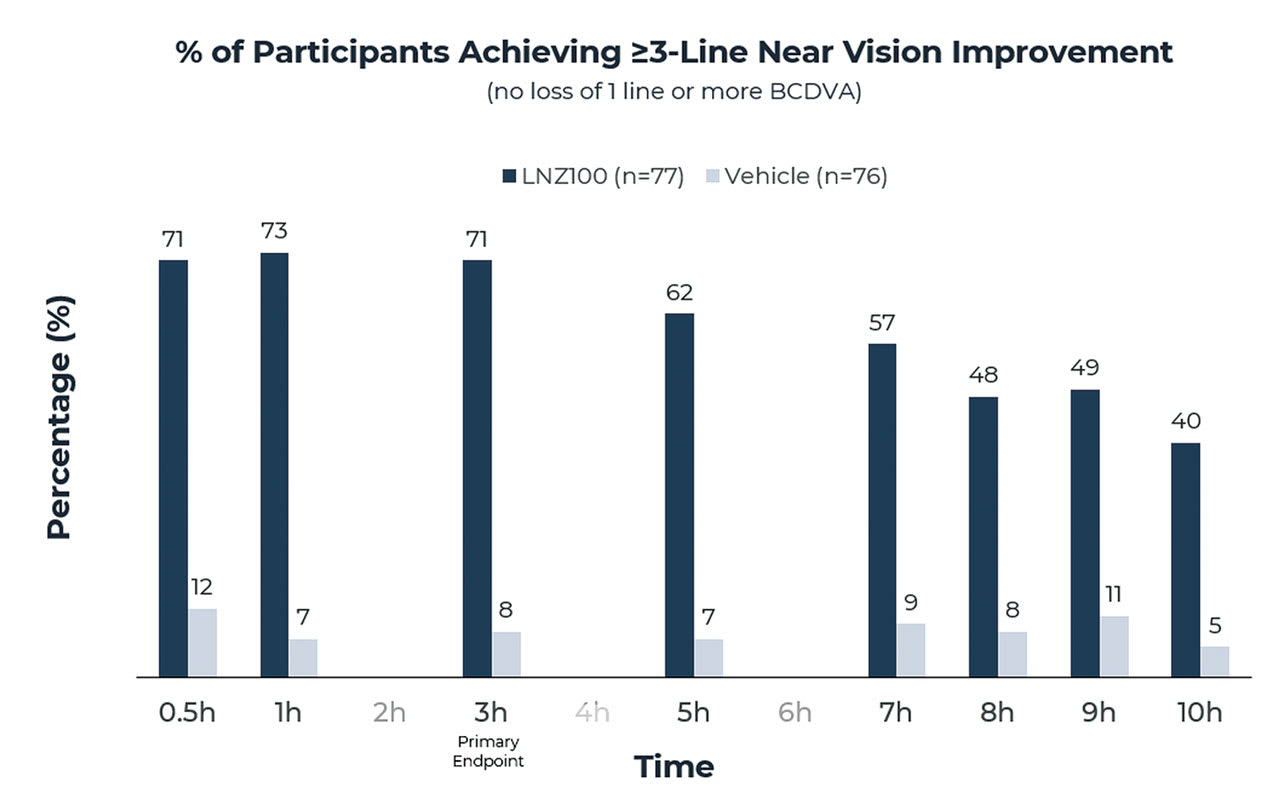
Results for LNZ100 from the CLARITY 2 trial utilizing the vehicle as a control (Figure 1) assessed improvement in monocular BCDVA at near without the loss of 1 line or more in BCDVA. The following were found:
• Rapid onset: 71% achieved 3 lines or greater improvement at 30 minutes.
• Primary endpoint: 71% achieved 3 lines or greater improvement at 3 hours.
• Long duration: 40% achieved 3 lines or greater improvement at 10 hours.
The vehicle control response rates were low, and the difference between LNZ100 and vehicle was highly significant at all time points measured (P<.0001).
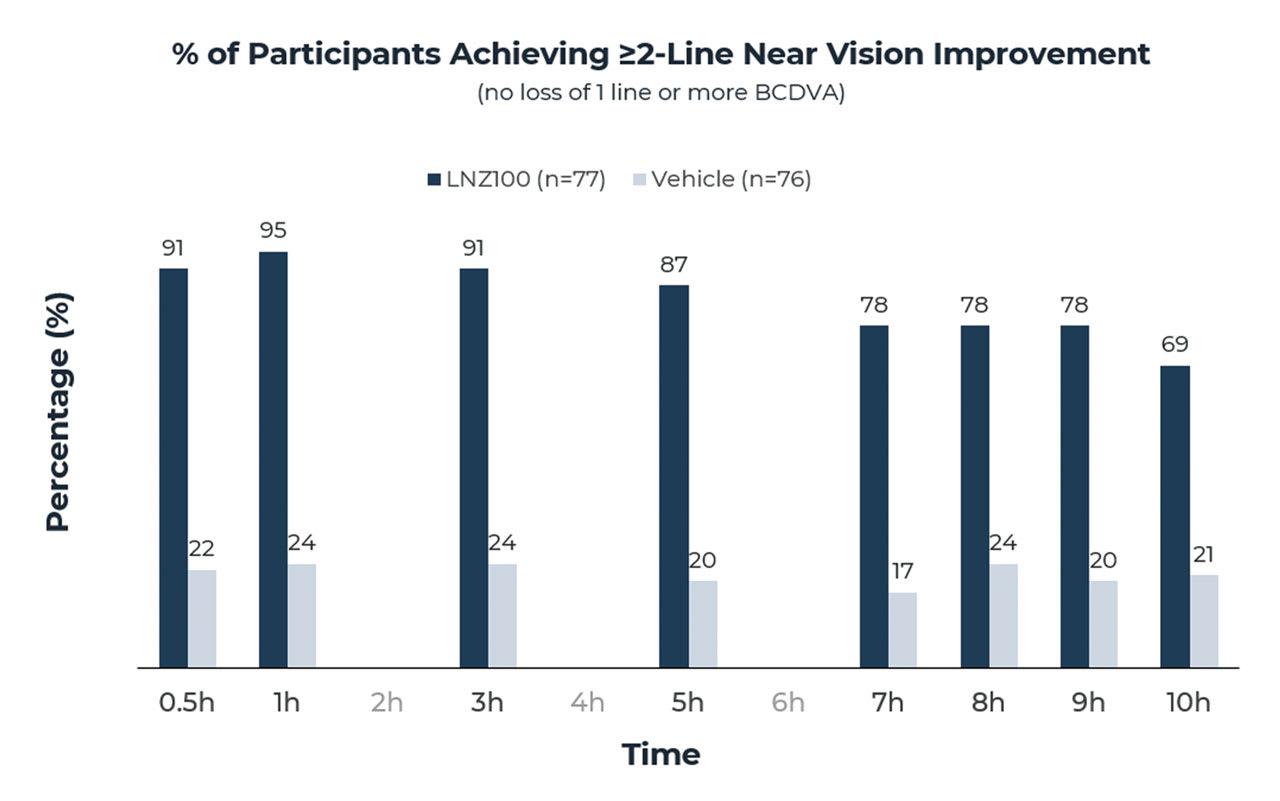
A secondary endpoint measurement of a 2-line or greater gain in monocular BCDVA at near without loss of 1 line or more of BCDVA was achieved in 95% of subjects at hour 1, and 69% of subjects had a 2-line or greater gain at 10 hours (Figure 2). A 2-line improvement in near vision is often considered a clinically relevant improvement that allows individuals to perform many important near vision tasks.
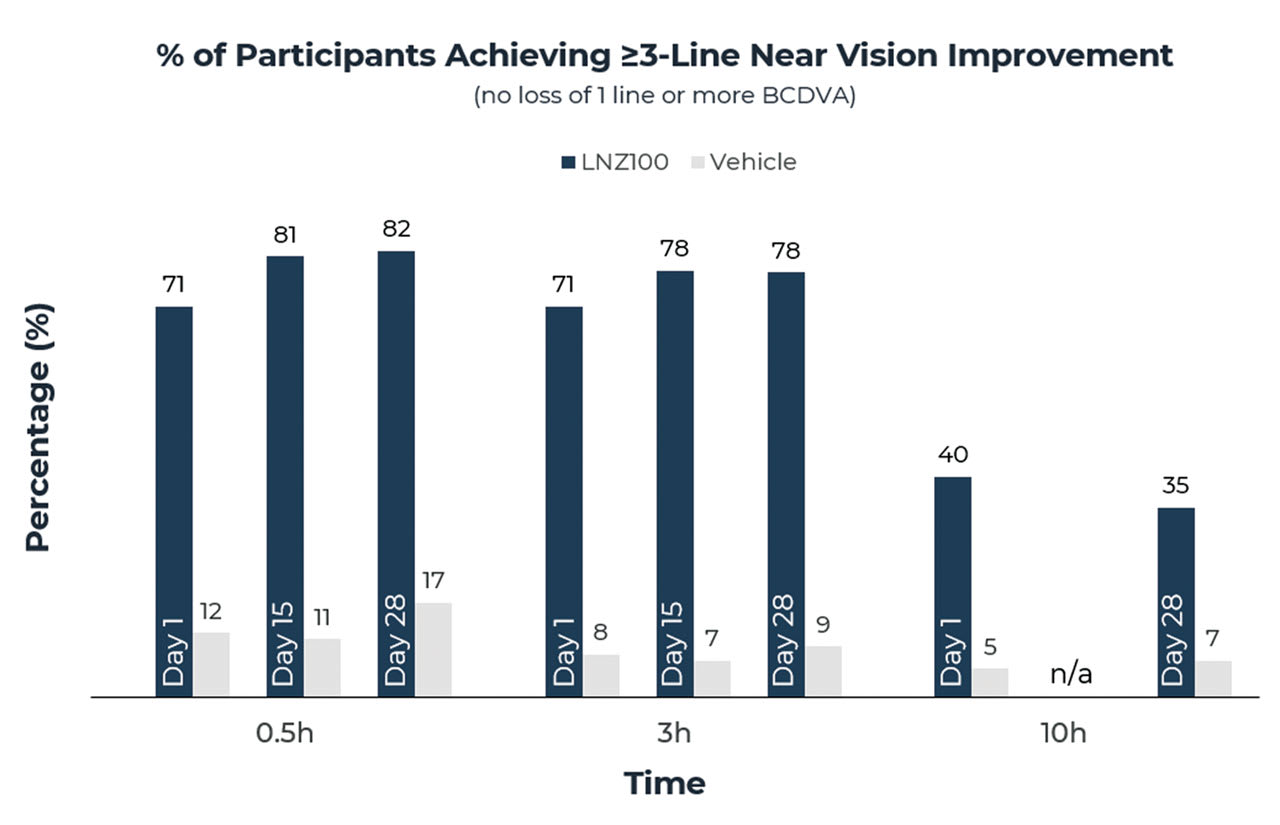
The near vision improvement found in the CLARITY 2 study was consistent across all study days at all time points measured on such study days (Figure 3).
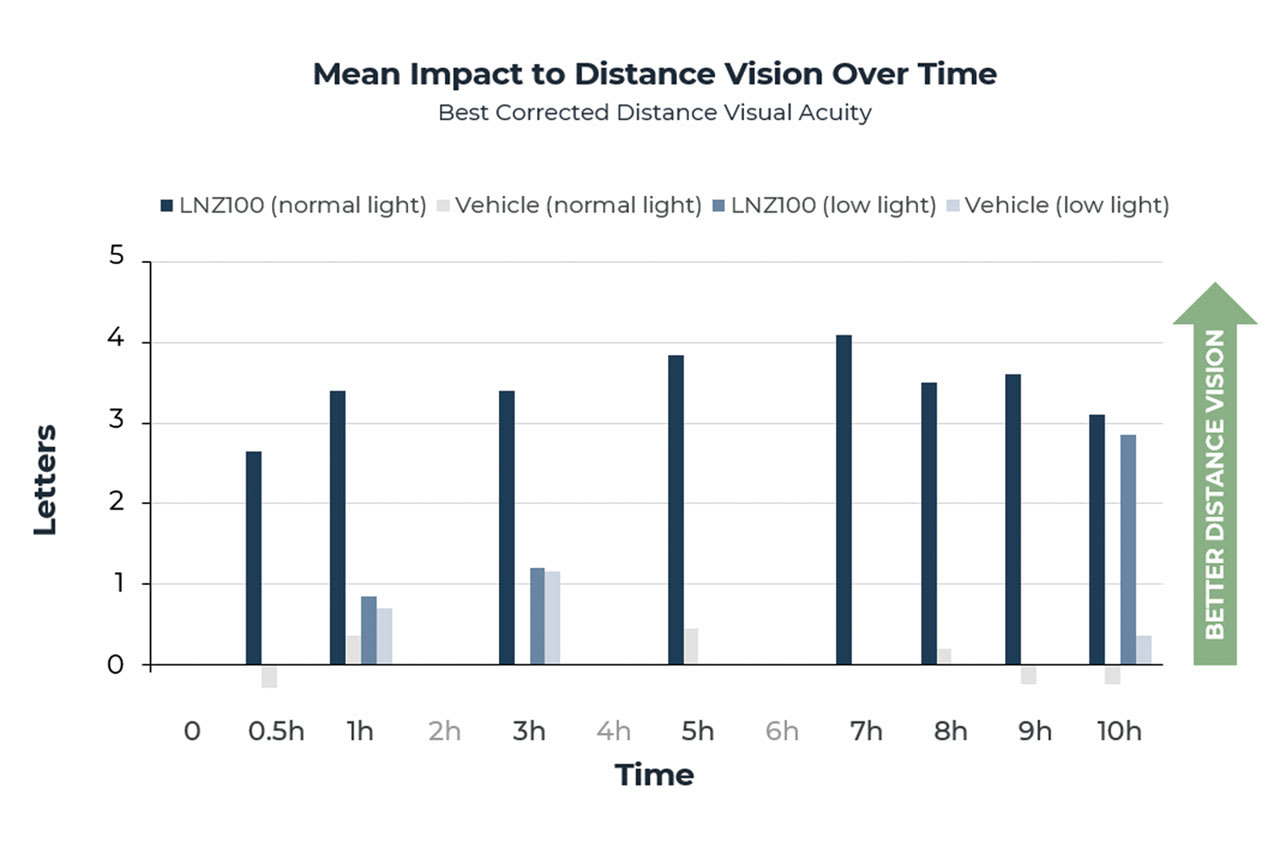
There was no negative impact on distance vision found in either normal- or low-illumination testing. In fact, a statistically significant improvement in BCDVA of 2 to 4 letters was found under normal lighting conditions at all time points compared to preadministration BCDVA (P<.0001) (Figure 4).
LNZ100 was well tolerated with no serious treatment-related adverse events (AEs) in the >30,000 treatment days across all 3 CLARITY trials (Figure 5). Ocular AEs that were reported in CLARITY 1 and 2 trials at a frequency of >5% were instillation site irritation (mild stinging upon instillation), visual impairment (mostly mild dimness), and hyperemia (mild eye redness). All of these reports were classified as mild. There was a vehicle-corrected combined frequency of headache reported in the CLARITY 1 and 2 trials of 7.6%, with the vast majority (89%) graded as mild.

Participant surveys further confirmed the commercial potential of LNZ100, with 90% of participants noticing an improvement in near vision and 75% of the participants indicating that they would continue to use LNZ100 after the study, of whom 81% expected to use the drop 4-7 days per week (Figure 6).
With the successful completion of the phase 3 trials with potential best-in-class data, Lenz is targeting FDA submission of LNZ100 in mid-2024, and there is great excitement about the promise of a pupil-selective miotic in development for presbyopia to address near vision needs in a new, unique, and effective manner.
References
1. Topline CLARITY results. Lenz Therapeutics website. Accessed May 8, 2024. https://ir.lenz-tx.com/news-events/presentations









