Presbyopia represents the single largest ophthalmic indication, affecting 2 billion people globally. Neither the conventional refractive approaches (glasses, contact lenses) nor myriad surgical options (monovision LASIK, corneal inlays, refractive intraocular lenses) have fully satisfied the needs of the presbyopic population. The first presbyopia-correcting topical eyedrop, a 1.25% pilocarpine drop (Vuity; AbbVie), was introduced in 2021 but has met with somewhat limited success, partly due to its short duration of effect. Three more presbyopia-correcting eyedrops are expected to be introduced in 2024-2025.
One of these drops is Brimochol PF (Visus Therapeutics), a once-daily, preservative-free, fixed-dose combination of a potent cholinergic miotic (carbachol 2.75%) and an alpha-2 agonist (brimonidine tartrate 0.1%).
Effectiveness
In BRIO-I, the first of 2 phase 3 clinical trials, Brimochol PF met all of its primary and secondary endpoints.1 The combination drug was administered to 182 emmetropic patients, ages 45-80, at 15 US sites. Subjects were randomized to a single dose of Brimochol PF, carbachol monotherapy, or brimonidine monotherapy, and then crossed over at subsequent visits to each of the other 2 study arms.
At 1, 2, 4, and 6 hours after the single dose, Brimochol PF was superior to carbachol and brimonidine monotherapies in the proportion of patients achieving a ≥15-ETDRS letter gain (3 lines) from baseline in binocular uncorrected near visual acuity under mesopic conditions, without a ≥5-ETDRS letter loss (1 line) in binocular uncorrected distance visual acuity—the primary endpoint established by the FDA for all studies of presbyopia-correcting drops. By demonstrating the statistical superiority of Brimochol PF to carbachol and brimonidine separately, and with Lenz Therapeutics recently reporting that its combination of aceclidine and brimonidine (LNZ-101) failed to demonstrate superiority over aceclidine alone (LNZ-100), Brimochol PF is the only eyedrop with data to support the efficacy of a combination product in phase 3 trials.
Another endpoint of interest is the proportion of patients who could see 20/40 or better at near since this outcome equates to near vision good enough to be glasses free for most tasks. For Brimochol PF, 85% of subjects could read 20/40 at near at 1 hour, 65% at 6 hours, and 56% at 8 hours. Even at 10 hours, nearly half the patients could still read 20/40 (Figure 1).

This 10-hour durability was achieved with just 1 drop, which sets this medication apart from Vuity and the soon-to-be-introduced Qlosi (pilocarpine 0.4%; Orasis Pharmaceuticals), both of which are twice-daily drops for most people. The target population for presbyopia-correcting drops is working adults in their 40s and 50s, and these patients say they would prefer a drop that lasts at least 7 hours.2 Although individual needs will vary, it makes sense that instilling drops in the morning and having them last all day would work well for many patients’ daily rhythms and visual demands. Further, it can be impractical to take a second drop while at work. In the BRIO-I study, the median score for patients reporting the duration of effect was “just right,” ie, neither too short nor too long, and they said they would use these drops approximately 5 out of 7 days.
The objective pupillometry data (Figure 2) from BRIO-I are also very reassuring. Not only do they validate the study’s subjective near vision results, but they also demonstrate that the miotic effects of Brimochol PF wear off very gradually throughout the day, helping to balance near vision improvement with minimizing nighttime vision risks. The study demonstrates that brimonidine makes a meaningful contribution to pupillary miosis because the combination therapy shows a clinically and statistically significant reduction in pupil size over carbachol alone at all time points from 30 minutes to 10 hours.

Safety
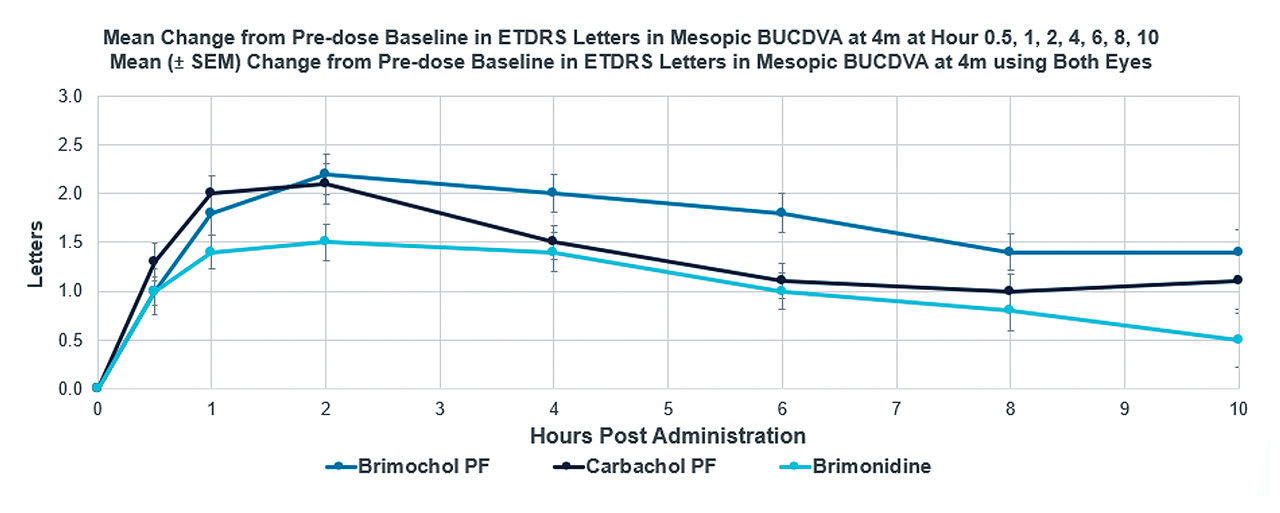
We have a long history of using both carbachol and brimonidine, so their safety profile is well established. In the BRIO-I study, there was no loss of distance vision in any of the 3 study arms. In fact, the Brimochol PF subjects experienced a small but statistically significant gain in uncorrected distance vision at all time points out to 8 hours (Figure 3).
There were no treatment-related serious adverse events and no discontinuations due to adverse events in the study. The most commonly experienced symptoms in the Brimochol PF arm of the study were transient eye irritation on instillation (14% of patients) and headache (9%), comparing quite favorably with recently reported adverse event rates for Qlosi and LNZ-100 (aceclidine 1.75%, Lenz Therapeutics).
Patients receiving Brimochol PF in BRIO-I reported no hyperemia at all, while the carbachol group did report redness, which is common with cholinergic agents due to the vasodilatory effects of cholinergic agents on the conjunctival vessels. We know that brimonidine, which is approved for use at a 0.025% concentration for relief of ocular redness (Lumify; Bausch + Lomb), has provided effective eye whitening without the side effects or rebound hyperemia of other vasoconstrictors.3,4
Next Steps
In a second phase 3 clinical trial, BRIO-II, the efficacy of Brimochol PF is being compared to vehicle, and safety with daily dosing is being evaluated over 12 months.5 With the data from this clinical trial expected in late 2024, Brimochol PF is likely to be the fourth presbyopia-correcting drop to market. Qlosi was recently approved and is expected to be commercially available soon, and LNZ100 has already completed phase 3 clinical trials. This time around, ther might be more interest and uptake because both optometrists and ophthalmologists have a better understanding of how these drugs work, how best to select patients, and how to monitor for adverse events. I personally feel that introduction of longer-acting presbyopia-correcting drops could reinvigorate interest in the category as well, because this is what my patients are asking for.
References
1. El-Harazi S, Evans DG, Williams JM, Schiffman R. Safety and Efficacy of Preservative-Free Carbachol 2.75%/Brimonidine Tartrate 0.1% Compared to Its Individual Components in Presbyopia. Poster presented at: American Society of Cataract and Refractive Surgery (ASCRS); April 5-8, 2024; Boston
2. Barnett M, Jasper AL. Patient motivations & expectations of presbyopia-correcting drops. Poster presented at: Annual meeting of the American Academy of Optometry; November 3-6, 2021; Boston.
3. McLaurin E, Cavet ME, Gomes PJ, Ciolino JB. Brimonidine ophthalmic solution 0.025% for reduction of ocular redness: a randomized clinical trial. Optom Vis Sci. 2018;95(3):264-271.
4. Lee JS, Kim CY. Brimonidine tartrate ophthalmic solution 0.025% for redness relief: an overview of safety and efficacy. Expert Rev Clin Pharmacol. 2022;15(8):911-919.
5. Clinicaltrials.gov. Safety and Efficacy Study of BRIMOCHOL™ PF and Carbachol PF in Subjects With Emmetropic Phakic and Pseudophakic Presbyopia. Available online: https://clinicaltrials.gov/study/NCT05135286 (accessed on 20 August 2024).
Disclosures: Dr. McCabe is a consultant for Visus and AbbVie, and has received research support from Orasis and Allergan.









